Climate Changes due to Ocean Acidification
Ocean Acidification
In the 200-plus years since the industrial revolution began, the concentration of carbon dioxide (CO2) in the atmosphere has increased due to humans burning fossil fuels (such as car emissions) and changing the way land is used (such as deforestation).
During this time, the pH of surface ocean waters has fallen by 0.1 pH units. The pH scale, like the Richter scale, is logarithmic, so this change represents approximately a 30 percent increase in acidity.
 |
| figure:1. Ocean Acidification |
The ocean absorbs about 30% of the CO2 that is released in the atmosphere, and as levels of atmospheric CO2 increase, so do the levels in the ocean. When CO2 is absorbed by seawater, a series of chemical reactions occur resulting in the increased concentration of hydrogen ions. This increase causes the seawater to become more acidic and causes carbonate ions to be relatively less abundant.
 |
| figure:2. Climate Changes Process |
Marine scientists are concerned that the process of ocean acidification constitutes a threat to sea life and to the cultures that depend on the ocean for their food and livelihood. Increases in ocean acidity reduce the concentration of carbonate ions and the availability of aragonite (a significant source of calcium carbonate) in seawater. Marine scientists expect that coral, shellfish, and other marine calcifiers (that is, organisms that use carbonates) will be less able to obtain the raw materials that they use to build and maintain their skeletons and shells. These scientists also note that rising ocean acidity presents a number of other physiological problems to different groups of marine organisms and that such problems could threaten the stability of marine food chains.
Effects of Ocean Acidification
Oceans absorb a substantial proportion of the CO2 emitted into the atmosphere by human activities, with potentially negative effects on shell-forming organisms.
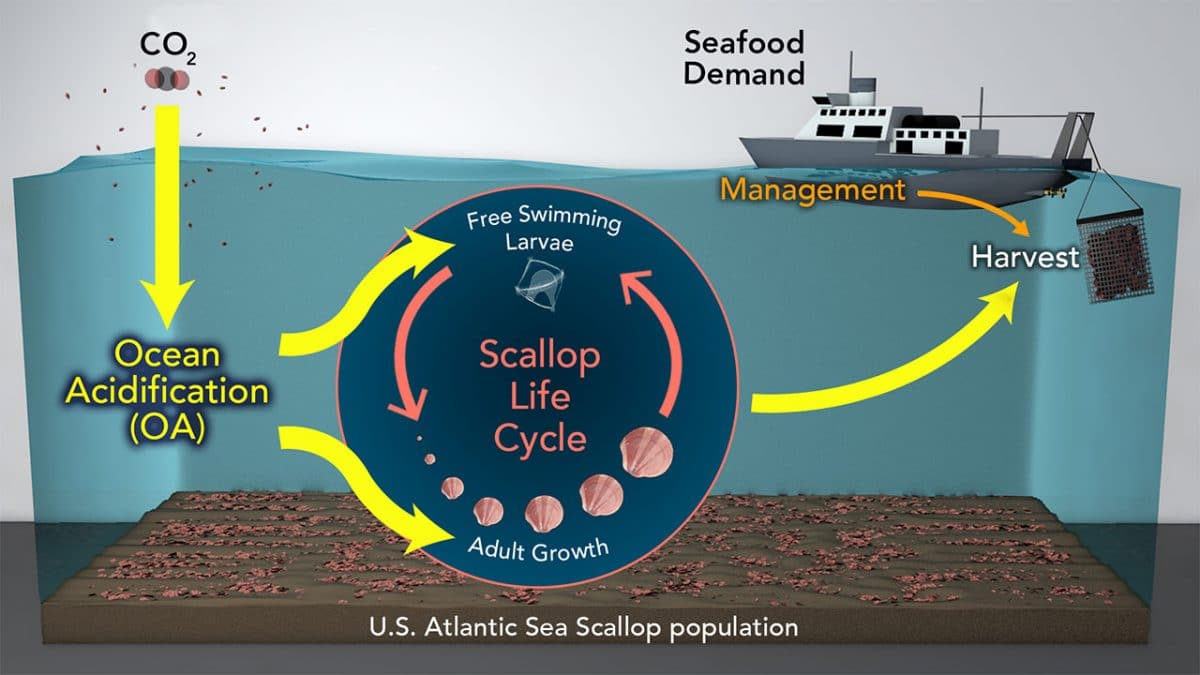 |
| figure:3. The Scallop life cycle |
- The oceans have absorbed between a third and a half of the CO2 humans have released into the atmosphere since about 1850. This has slowed the rate of climate change.
- The oceans have absorbed between a third and a half of the CO2 humans have released into the atmosphere since about 1850. This has slowed the rate of climate change.
- When CO2 dissolves in seawater, the water becomes more acidic. The acidity of the oceans has increased by 26 % since about 1850, a rate of change roughly 10 times faster than any time in the last 55 million years.
- Associated chemical reactions can make it difficult for marine calcifying organisms, such as coral and some plankton, to form shells and skeletons, and existing shells become vulnerable to dissolution.
- The extent to which calcifying organisms are already being affected by acidification is unclear, as this is a very new area of study. Limited evidence suggests that some organisms are more sensitive than others.
- The rate at which acidification occurs is a determining factor in the extent to which calcifying organisms will be able to adapt.
- The impacts of acidification will extend up the food chain to affect economic activities such as fisheries, aquaculture and tourism. Wherever there are marine calcifying organisms, there are risks from ocean acidification.
The Effects on Human Societies
- Food: Ocean acidification has the potential to affect food security. Commercially and ecologically important marine species will be impacted, although they may respond in different ways. Molluscs such as oysters and mussels are among the most sensitive groups.
- Coastal protection: Marine ecosystems such as coral reefs protect shorelines from the destructive action of storm surges and cyclones, sheltering the only habitable land for several island nations. This protective function of reefs prevents loss of life, property damage, and erosion, and has been valued at US$9 billion per year.
- Tourism: This industry could be severely affected by the impacts of ocean acidification on marine ecosystems (e.g. coral reefs). In Australia, the Great Barrier Reef Marine Park attracts about 1.9 million visits each year and generates more than A$5.4 billion to the Australian economy.
- Carbon storage and climate regulation: The capacity of the ocean to absorb CO2 decreases as ocean acidification increases. More acidic oceans are less effective in moderating climate change.
Steps to Prevent Ocean Acidification
At the local level, the following policy and management options can help to minimise the adverse effects of other local stressors and, as a result, help marine ecosystems to cope better with changing environmental conditions.
 |
| figure:4. The silencing of the seas: |
- Improvements in water quality: Monitoring and regulating localised sources of acidification from runoff and pollutants such as fertilisers.
- Development of sustainable fisheries management practices: Regulating catches to reduce overfishing and creating long-term bycatch reduction plans.
- Implementation of new technologies: Different techniques can be applied depending on the industry. For example, in the aquaculture industry, new forecasting systems have been developed to account for seasonal upwellings that bring low pH seawaters to the ocean surface and cause massive shellfish die-offs.
- Sustainable management of habitats: Increasing coastal protection, reducing sediment loading and applying marine spatial planning.
- Establishment and maintenance of Marine Protected Areas: Protecting highly vulnerable and endangered marine ecosystems.
Thankyou for Reading...
vicky nagar

Nice work bro
ReplyDeletethanks bro
DeletePehle mujhe climate changes ke bare me kuchh pta nhi tha but jab se maine tera ye blog pdha...ab mai mausam vibhag me hoon....tumne meri zindagi badal di
ReplyDeleteaapka bhot bhot dhanyawad jo aapne mera blog padha, aur me khushkhismat hun jo mein aapki zindagi bdal skaa
DeleteNice ppt
ReplyDeleteNyc
ReplyDeleteGood work
ReplyDeletethanks
DeleteNice
ReplyDeleteGood work
ReplyDeletethanks
DeleteNice
ReplyDeletethanks
DeleteGood
ReplyDeleteGreat job Brother 👍
ReplyDelete